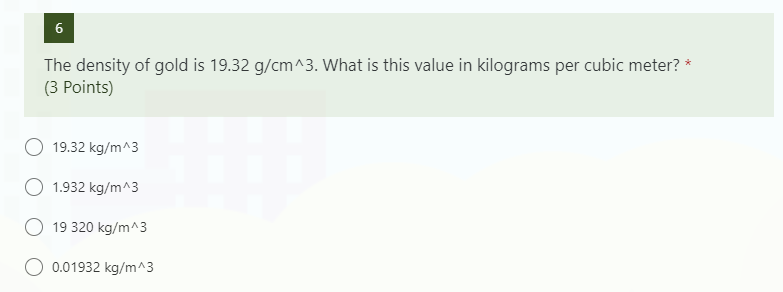
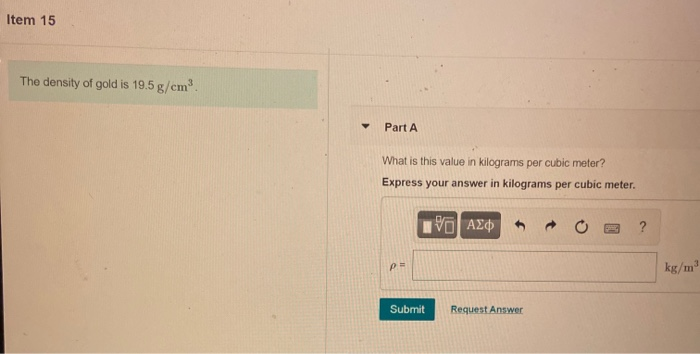
What kind of height and strength does a strong man need to hand over a tree? The mystery of MineCraft science – Hahapy
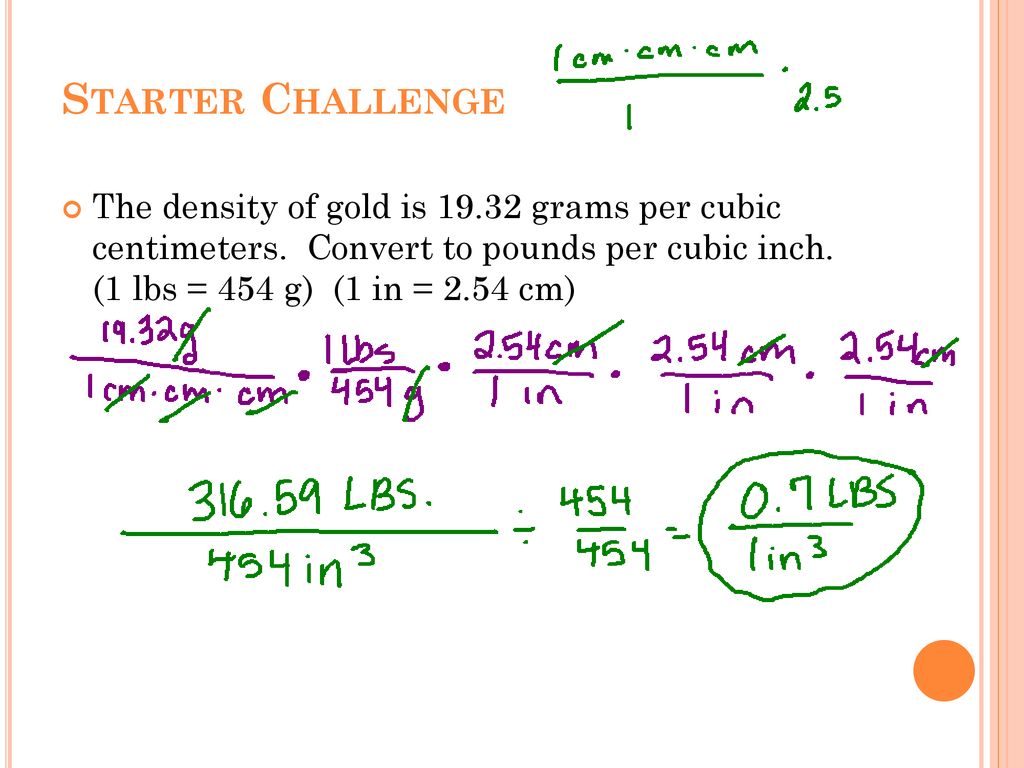
Starter Challenge The density of gold is grams per cubic centimeters. Convert to pounds per cubic inch. (1 lbs = 454 g) (1 in = 2.54 cm) - ppt download

A piece of pure gold of density `19.3 g cm^(-3)` is suspected to be hollow inside. It weighs 38.250 - YouTube

a) Calculate the number of free electrons per cubic meter for gold assuming that there are 1.5 free - Brainly.com

HW3_solutions - 4.4 Calculate the number of vacancies per cubic meter in gold (Au) at 900C. The energy for vacancy 3 formation is 0.98 eV/atom. | Course Hero

SOLVED: Boltzmann constant; k = 8.62 X 10*eVlatom K, Avogadro $ constant;, NAv:= 6.022 x 1023 mol Calculate the equilibrium number of vacancies per cubic meter in gold (Au) at 258C For



:max_bytes(150000):strip_icc()/GettyImages-139820160-58b599a75f9b5860467cbd20.jpg)